Which Statement Best Describes an Oxidation Reduction Reaction
A chemical reaction in which electrons are released from the system D. Which of the following statements best describes an ionic bond.

Calculating Molar Solubility Molarity High School Chemistry Solubility Chemistry Teacher
B Electrochemistry is the study of only reduction reactions.

. 4 What is the reducing agent in this reaction. A chemical reaction in which there are fewer products than reactants B. 1Which statement best describes an oxidation-reduction reaction.
The correct statement that describes a Redox reaction is D. The type of reaction that is shown is. 1Which statement best describes an oxidation-reduction reaction.
A chemical reaction in which electrons are released from the system. 4Al 302 2Al2O3 In this reaction aluminum is oxidized and oxygen is reduced In this reaction aluminum is reduced and oxygen is oxidized In this reaction th aluminum and oxygen are oxidized This is not an. The presence of which reactant is the best indicator of an oxidation-reduction reaction.
In a redox reaction an electron is lost by the reducing agent. Which identifies an oxidation-reduction reaction. So here reducing agent is a substance a substance that reduces other and oxidized itself.
Mg Mg2 2e D. Which statement best describes the relationship between electrochemistry and oxidation-reduction reactions. A chemical reaction that involves oxygen C.
Which statement best describes the oxidizing and reducing abilities of the reactants. Nadh is oxidized as a pair of electrons which are transferred to the etc and 4 four h are pumped into the intermembrane space. B The reaction occurs in a voltaic cell and absorbs energy.
A The reaction occurs in a voltaic cell and releases energy. Which answer best describes what is happening in the following reaction. These reactions involving electron transfers are known as oxidation-reduction or redox reactions.
Silver ion Ag is a stronger oxidizing agent than copper ion Cu2 and copper metal is a stronger reducing agent than silver. A Redox reaction oxidation-reduction reaction involves the exchangetransfer. Fe Cu2 Fe2 Cu A Two electrons are lost.
B Electrochemistry is the study of only reduction reactions. Which of the following statements best describes how a reducing agent in is chemically altered in a biological redox reaction. Because of this in many cases H 2 O or a fragment of an H 2 O molecule H or OH in particular can participate in the redox reactionAs such we need to learn how to incorporate the solvent into a balanced redox equation.
A chemical reaction in which there are fewer products than reactants. A Electrochemistry is the study of only oxidation reactions. This is a redox reaction in which octane C8H18 is oxidized.
Behave given christian S here which of the following statements best describes how our reducing agent in is chemically altered in a biologically dogs reaction. O lt gains a hydrogen atd and gains potential energy. 5 Which identifies an oxidation-reduction reaction.
The sharing of a pair of electrons between two atoms a relatively weak bond. Olt gains a hydrogen atom and loses potential energy O It loses a hydrogen atom and loses potential energy It loses a hydrogen atom and gains potential energy. If there is formation of a precipitate the reaction is an oxidation-reduction reaction.
In this reaction iron is reduced and copper is oxidized In this reaction both iron and copper are oxidized Which statement best describes the following reaction. Which statement best describes what changes occur over the course of the following oxidation-reduction reaction. C decomposition reaction.
C Oxidation-reduction reactions are the basis of electrochemical cells. 1 Which statement best describes what is taking place in this half reaction Fe. The reaction that takes place in a chemical cell is best classi ed as A.
When an element is oxidized it loses electrons. So reducing agent is a substance that produces others and oxidize itself. Which equation represents the half-reaction that takes place at.
This is a redox reaction in which octane C8H18 is oxidized. In redox reactions a reduced half and an oxidized half occur together. Chemistry 25022021 1840 olivia0420.
Cl2 2e 2Cl C. Which statement best describes what happens during the redox reaction between nadh and complex i of the electron transport chain. When an object is electroplated the occurrence of a redox reaction is nonspontaneous and it requires an electric current.
Which statement best describes the reaction represented by the equation2NaCl 2H2O electricity -- Cl2 H2 2NaOH. B Iron changes into copper. Third option is the correct one.
Chemistry 19092021 2310 NetherisIsTheQueen. A chemical reaction in which electrons are released from the system D. 3 on a question.
6 In what order are redox reactions. 3 Which one of these reactions is not an oxidation-reduction reaction. Nadh is oxidized by fadh2 which is then immediately oxidized in complex complex ii.
1Which statement best describes an oxidation-reduction reaction. Which statement best describes the relationship between electrochemistry and oxidation-reduction reactions. A chemical reaction in which there are fewer products than reactants B.
A Electrochemistry is the study of only oxidation reactions. A chemical reaction in which electrons are transferred between reactants. The attraction between two charged atoms a relatively weak bond in an aqueous solution.
A chemical reaction in which electrons are transferred between reactants 2Beryllium Be has four electrons. A chemical reaction that involves oxygen C. However an oxidizing agent oxidizes something else and gets reduced therefore gaining electrons.
So here first we will discuss that What is reducing agent. The sharing of a pair of electrons between two atoms a relatively strong bond. A chemical reaction that involves oxygen.
2 What is the overall equation for the chemical reaction Zn. C The reaction occurs in an electrolytic cell and releases energy. Which answer best describes what is happening in the following reaction.
Which of the following is true about a redox reaction. A chemical reaction in which electrons are transferred between reactants. An oxidation-reduction redox reaction is a type of chemical reaction in which electrons are transferred between chemical species.
D Copper transfers two electrons to iron. But when an element is reduced it gains electrons. O O O If there is a change in the oxidation states of atorns from reactants to products the reaction is an oxidation- reduction reaction If there is formation of salt and water the reaction is an oxidation-reduction reaction.
C Iron transfers two electrons to copper. The chemical formula that shows the correct subscripts is D BeF₂. In the reaction MgCl2MgCl2 the correct half-reaction for the oxidation that occurs is A.
E Two electrons are gained. This is the basis of redox reactions.
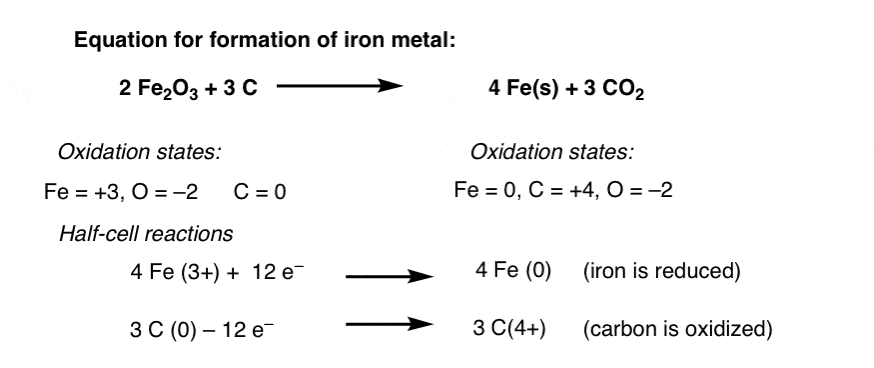
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry

Question Of The Day In 2020 This Or That Questions Question Of The Day Coaching

Rbse Solutions For Class 12 Chemistry Chapter 5 Surface Chemistry Rbsesolutionsforclass12chemistry Rbsesolutions Chemistry Physical Chemistry Solutions

Chemistry Reduction And Oxidation Reactions Wikiversity
Is Cellular Respiration An Oxidation Or Reduction Reaction
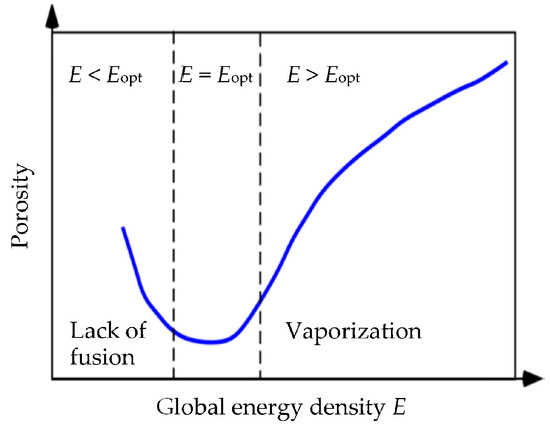
Materials Free Full Text Modeling Of Processing Induced Pore Morphology In An Additively Manufactured Ti 6al 4v Alloy Html

Glycoprotein Vs Proteoglycan Contain Glycosaminoglycans Biochemistry Biochemical Medical Science
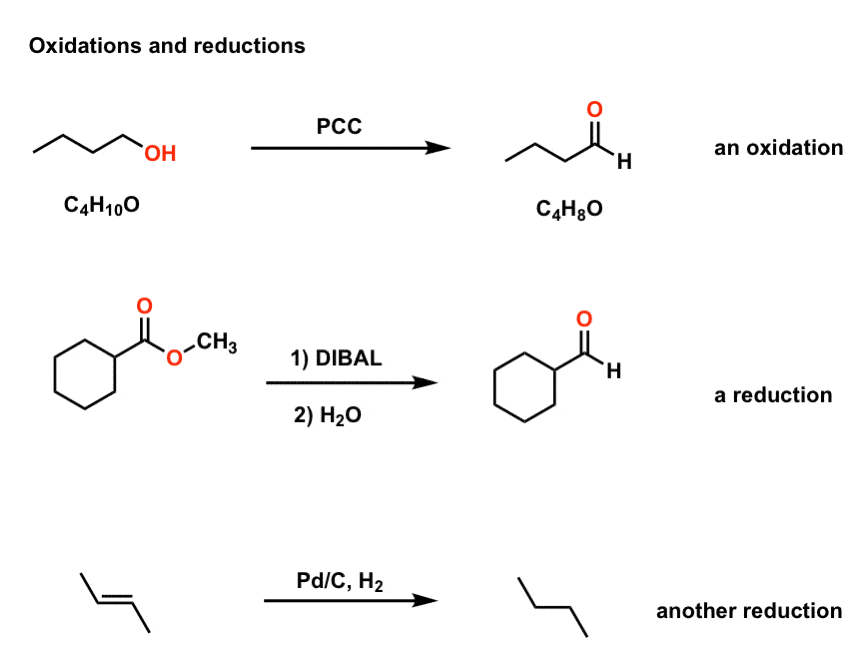
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry

Using Oxidation Numbers To Identify Oxidation And Reduction Worked Example Video Khan Academy

1 Which Statement Best Describes An Oxidation Reduction Reaction 1 Point A A Chemical Reaction In Brainly Com

Electrolysis The Way Of The Future Electrochemistry Chemical Reactions Chemical Analysis

Bioluminescence Bioluminescent Algae Bioluminescent Algae Bioluminescence Types Of Lighting

Image Result For Toothbrush Circuit Charger Mains Contactless Brushing Teeth Solar Power Charger
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry

Modeling Of Steelmaking Processes 1st Edition Ebook Rental In 2021 Ebook Model Crc Press

Oxidation Reduction Reactions Boundless Chemistry

Usgs Fact Sheet Fs109 03 Fact Sheet Sulphur Cycle Groundwater

Resumen Del Ciclo De Calvin Benson Paperblog Photosynthesis Photosynthesis And Cellular Respiration Cellular Respiration

Comments
Post a Comment